HCN Lewis Structure Hydrogen cyanide, commonly known as HCN, is a simple yet extremely important molecule in chemistry. Despite having only three atoms—hydrogen, carbon, and nitrogen—HCN plays a crucial role in understanding chemical bonding, molecular geometry, and electron distribution. One of the best ways to visualize and understand this molecule is through the HCN Lewis structure, which shows how valence electrons are arranged and how atoms bond with each other. For students, especially those studying basic chemistry, Lewis structures serve as the foundation for more advanced topics like molecular orbital theory and reaction mechanisms.
The HCN Lewis structure is widely discussed in academic courses because it includes a triple bond, formal charge considerations, and a linear molecular shape. Learning how to correctly draw and analyze this structure helps students master the octet rule, understand electron sharing, and predict molecular behavior. In exams and competitive tests, questions related to HCN often appear due to its conceptual clarity and relevance.
Understanding the HCN Lewis structure also bridges the gap between theory and application. From explaining molecular polarity to predicting chemical reactivity, this structure provides insights that are essential for both classroom learning and real-world chemistry. In this article, we will explore every aspect of the HCN Lewis structure in a clear, step-by-step, and student-friendly manner.
Understanding the Basics Before Drawing HCN Lewis Structure
Before drawing the HCN Lewis structure, it is essential to understand the basic components of the molecule. Hydrogen cyanide consists of one hydrogen atom, one carbon atom, and one nitrogen atom. Each of these atoms contributes a specific number of valence electrons, which are the electrons involved in bonding. Hydrogen contributes one valence electron, carbon contributes four, and nitrogen contributes five, giving a total of ten valence electrons in the HCN molecule.
Another key concept to understand is the octet rule. According to this rule, most atoms tend to bond in such a way that they have eight electrons in their outer shell, achieving stability similar to noble gases. Hydrogen is an exception and follows the duet rule, meaning it is stable with just two electrons. Carbon and nitrogen in HCN both aim to complete their octets through shared electrons.
Electronegativity also plays a role in determining how atoms bond in HCN. Carbon is less electronegative than nitrogen but more electronegative than hydrogen, making it the ideal central atom. Understanding these basic principles ensures that when you draw the HCN Lewis structure, it is both chemically accurate and stable. These fundamentals form the backbone of all Lewis structure drawings.
Step-by-Step Method to Draw HCN Lewis Structure
Drawing the HCN Lewis structure becomes straightforward when approached systematically. The first step is counting the total number of valence electrons. As discussed earlier, hydrogen provides one electron, carbon four, and nitrogen five, giving a total of ten valence electrons that must be placed in the structure.
The next step is identifying the central atom. In the HCN Lewis structure, carbon is placed at the center because it can form multiple bonds and has moderate electronegativity. Hydrogen and nitrogen are placed on either side of carbon, forming a linear skeletal structure: H–C–N. Once the skeletal structure is in place, single bonds are drawn between hydrogen and carbon, and between carbon and nitrogen. Each single bond represents two shared electrons.
After placing single bonds, the remaining electrons are distributed to complete the octets of the atoms. Hydrogen already satisfies its duet rule, but carbon and nitrogen still need more electrons. To fulfill the octet rule, two additional bonds are formed between carbon and nitrogen, resulting in a triple bond. This final step ensures all atoms are stable, completing the correct HCN Lewis structure.
Electron Dot Diagram of HCN Lewis Structure
The electron dot diagram is a visual representation of the HCN Lewis structure that clearly shows bonding and non-bonding electrons. In this diagram, shared electron pairs are represented as dots or lines between atoms, while lone pairs appear as dots around the atoms. For HCN, the diagram highlights the strong triple bond between carbon and nitrogen.
In the HCN Lewis structure, hydrogen shares one pair of electrons with carbon, forming a single bond. Carbon, acting as the central atom, shares three pairs of electrons with nitrogen, forming a triple bond. Nitrogen also has one lone pair of electrons, which plays a role in determining the molecule’s polarity and reactivity. Carbon does not have any lone pairs in this structure.
The electron dot diagram is especially useful for beginners because it visually reinforces the concept of electron sharing. By examining the HCN Lewis structure through an electron dot diagram, students can easily understand how electrons are arranged and why the molecule is stable. This visual clarity makes it easier to transition to advanced concepts like hybridization and molecular orbitals.
Formal Charge Analysis of HCN Lewis Structure
Formal charge is an important concept used to evaluate the stability of a Lewis structure. In the HCN Lewis structure, calculating formal charges helps confirm whether the drawn structure is the most stable arrangement of electrons. The formal charge is calculated using the formula:
Formal Charge = Valence Electrons – (Non-bonding Electrons + ½ Bonding Electrons).
When applied to hydrogen in HCN, the formal charge is zero because hydrogen has one valence electron and shares one bond. Carbon also has a formal charge of zero, as it effectively shares four electrons through bonding. Nitrogen, despite having a lone pair, also ends up with a formal charge of zero. This balance of formal charges indicates that the HCN Lewis structure is highly stable.
A structure with zero formal charges on all atoms is generally the most preferred. This confirms that the triple-bonded arrangement between carbon and nitrogen is not only correct but also the most energetically favorable. Formal charge analysis adds confidence to the accuracy of the HCN Lewis structure and is often tested in exams.
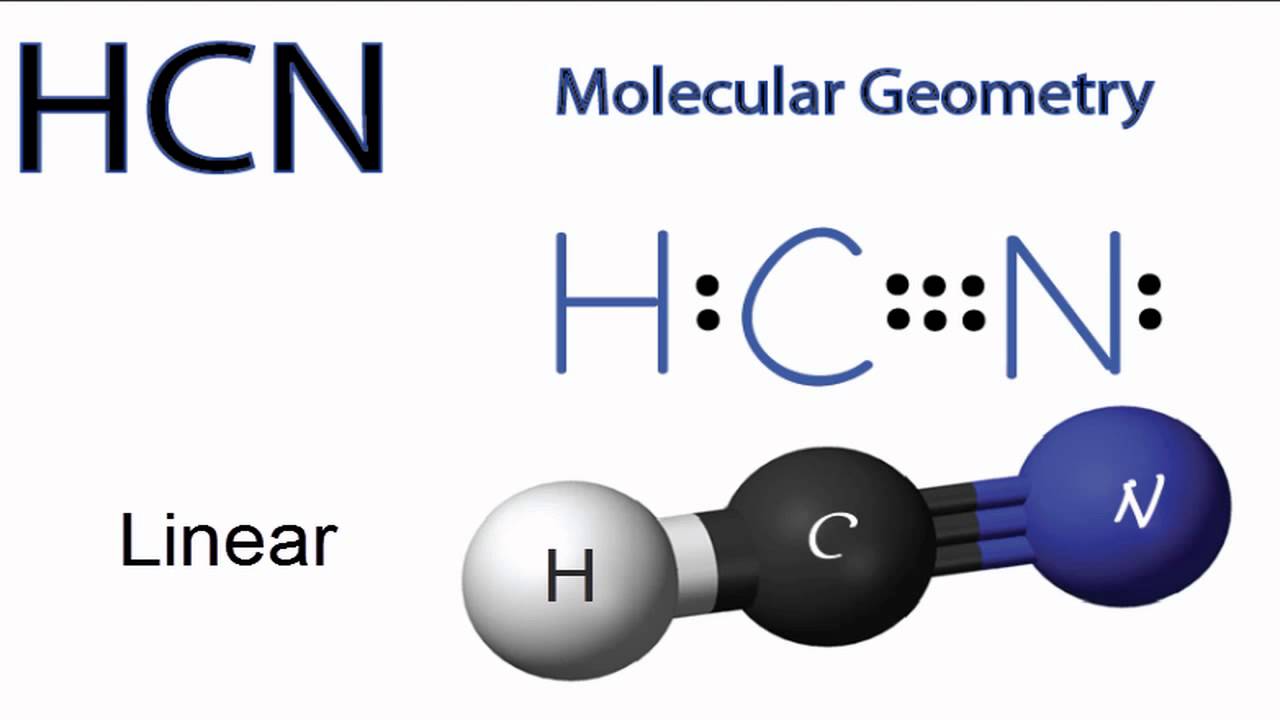
Molecular Geometry and Shape of HCN
The molecular geometry of HCN can be predicted using the Valence Shell Electron Pair Repulsion (VSEPR) theory. According to this theory, electron pairs around a central atom arrange themselves as far apart as possible to minimize repulsion. In the HCN Lewis structure, carbon has two regions of electron density: one single bond and one triple bond.
Because there are only two regions of electron density around the central carbon atom, the molecule adopts a linear geometry. This means the bond angle between hydrogen, carbon, and nitrogen is approximately 180 degrees. The linear shape contributes to the molecule’s polarity and physical properties.
Understanding the molecular geometry of HCN helps explain its chemical behavior. The linear shape allows for efficient overlap of orbitals, leading to strong bonding, especially in the carbon-nitrogen triple bond. This structural insight is a direct result of analyzing the HCN Lewis structure.
Hybridization in HCN Lewis Structure
Hybridization explains how atomic orbitals mix to form new hybrid orbitals suitable for bonding. In the HCN Lewis structure, carbon undergoes sp hybridization because it forms two regions of electron density. One sp orbital forms a sigma bond with hydrogen, while the other forms a sigma bond with nitrogen.
Nitrogen in HCN is also sp hybridized. It uses one sp orbital to form a sigma bond with carbon, while the remaining p orbitals form pi bonds that complete the triple bond. The presence of one lone pair on nitrogen occupies the remaining sp orbital.
Hybridization helps explain the linear shape and strong bonding in HCN. By understanding hybridization in the HCN Lewis structure, students gain a deeper appreciation of how molecular shape and bonding strength are interconnected.
Polarity of HCN Molecule
Polarity is determined by differences in electronegativity and molecular geometry. In the HCN Lewis structure, both bonds—H–C and C≡N—are polar due to differences in electronegativity. Nitrogen is significantly more electronegative than both carbon and hydrogen, pulling electron density toward itself.
Because HCN is linear and the electronegativity difference is not symmetrical, the dipole moments do not cancel out. As a result, HCN is a polar molecule with a net dipole pointing toward the nitrogen atom. This polarity affects its solubility, boiling point, and chemical reactivity.
Understanding polarity through the HCN Lewis structure is essential for predicting how the molecule interacts with other substances. It also explains why HCN is highly reactive and toxic, despite its simple structure.
Resonance Structures of HCN
Resonance occurs when more than one valid Lewis structure can be drawn for a molecule. In the case of HCN, the dominant structure features a triple bond between carbon and nitrogen. While alternative resonance forms can be proposed, they involve unfavorable formal charges.
Because the primary HCN Lewis structure has zero formal charges and satisfies the octet rule, it is the most stable and significant representation. Other resonance forms contribute very little to the overall structure.
This makes HCN a good example of a molecule with limited resonance, helping students understand when resonance is important and when a single Lewis structure is sufficient.
Common Mistakes While Drawing HCN Lewis Structure
One common mistake is choosing the wrong central atom. Placing hydrogen or nitrogen in the center leads to incorrect structures. Another frequent error is miscounting valence electrons, which can result in incomplete octets or extra electrons.
Students also sometimes forget to form the triple bond between carbon and nitrogen, leaving the molecule unstable. Ignoring formal charge analysis is another mistake that can lead to incorrect conclusions about stability.
Being aware of these mistakes helps learners draw the HCN Lewis structure accurately and confidently, especially during exams.
Conclusion
The HCN Lewis structure is a powerful learning tool that combines simplicity with depth. By understanding its valence electrons, bonding, geometry, hybridization, and polarity, students can grasp fundamental chemical concepts that apply to countless other molecules. This structure demonstrates how careful electron placement leads to molecular stability.
Mastering the HCN Lewis structure builds confidence in drawing Lewis structures and prepares learners for more advanced chemistry topics. Its clarity and relevance make it an essential example in chemical education.
Frequently Asked Questions
What is the correct Lewis structure of HCN?
The correct structure is H–C≡N, with a triple bond between carbon and nitrogen.
Why does HCN have a triple bond?
The triple bond helps carbon and nitrogen complete their octets.
Is HCN linear or bent?
HCN is linear with a bond angle of 180 degrees.
How many valence electrons are in HCN?
HCN has a total of ten valence electrons.
Is HCN a polar molecule?
Yes, HCN is polar due to electronegativity differences.
What is the hybridization of carbon in HCN?
Carbon is sp hybridized in HCN.
Does HCN follow the octet rule?
Yes, carbon and nitrogen both satisfy the octet rule.
Are there resonance structures for HCN?
There are minor resonance forms, but the main structure dominates.
Also Read: Dodge Charger Scat Pack